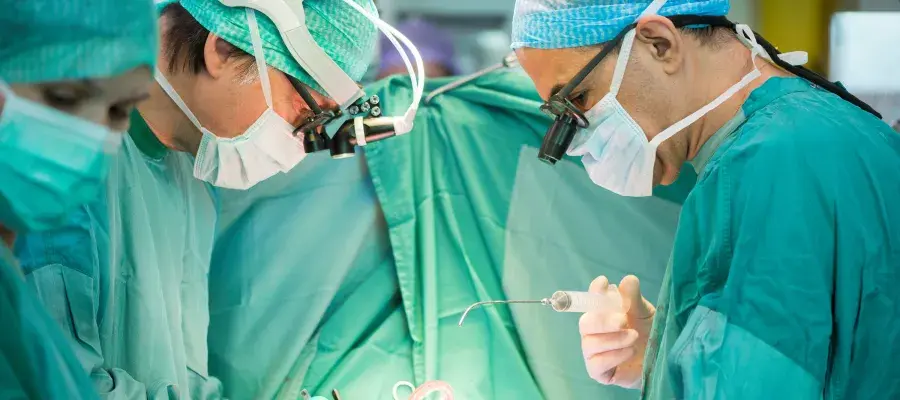
Heart bypass surgery remains a cornerstone treatment for coronary heart disease, offering a lifeline to patients by rerouting blood flow around blocked arteries. However, a significant challenge persists: the long-term failure of the vein grafts used in the procedure. A pioneering clinical trial, led by researchers at the University of Edinburgh in collaboration with NHS Greater Glasgow and Clyde and the University of Glasgow, is now exploring an innovative solution to this problem through cardiac gene therapy. This landmark study aims to enhance the durability of these grafts, potentially improving patient outcomes for years to come.
Addressing the Challenge of Vein Graft Failure
During a standard heart bypass operation, surgeons typically use a combination of one artery and two or more veins to create new pathways for blood circulation. While this procedure is life-saving, the vein grafts are not naturally designed to handle the high-pressure environment of the arterial system. Over time, this can lead to a process called intimal hyperplasia, where the vessel wall thickens and narrows, ultimately causing the graft to fail. This failure can necessitate further surgeries or lead to serious cardiovascular events.
The PROTECT study is at the forefront of addressing this issue. The trial investigates a novel gene therapy designed to be applied directly to the vein segment before it is grafted into the heart. By modifying the genetic material of the vein, the therapy aims to bolster its resilience against the stresses of its new role in the circulatory system.
A Groundbreaking Therapeutic Approach
The core of this innovative treatment involves the precise delivery of a gene that produces a protein known as TIMP-3 (Tissue Inhibitor of Metalloproteinases 3). This protein plays a crucial role in tissue remodeling and has been shown to help prevent the thickening and blockage of blood vessels. By introducing the TIMP-3 gene into the vein graft, researchers hope to maintain its patency and function over a longer period.
A key innovation of the trial is the method of delivery. The research team has developed a technique to treat the vein at the time of surgery, ensuring the gene therapy is administered safely and efficiently just before the graft is put into place. This targeted, just-in-time approach represents a significant step forward in the practical application of gene therapy in a surgical setting.
The Collaborative Effort Behind the Trial
This ambitious clinical trial is a testament to extensive interdisciplinary collaboration. It is led by NHS Greater Glasgow and Clyde and the University of Glasgow, working in close partnership with NHS Golden Jubilee and the University of Edinburgh. The study has received substantial backing from major funding bodies, including the Medical Research Council (MRC) and the British Heart Foundation (BHF).
Professor Colin Berry of the University of Glasgow highlighted the long-term nature of this research, stating that the new gene therapy is the result of more than two decades of teamwork. Professor Andrew Baker, the academic lead for the study from the University of Edinburgh, emphasized the importance of this collaboration in translating a laboratory discovery into a clinical reality. The project also benefits from the support of the Cell and Gene Therapy Catapult and other advanced therapy treatment centers across the UK.
Looking to the Future of Cardiac Care
The successful application of this gene therapy could have a profound impact on the future of heart bypass surgery. If the trial demonstrates that the TIMP-3 gene therapy can effectively extend the lifespan of vein grafts, it could significantly improve the long-term health of patients, reduce the need for repeat surgeries, and ultimately save lives. This research underscores the critical role of sustained investment in cardiovascular science and the potential for advanced therapies to solve long-standing clinical challenges.
As the first patient has now been treated, the medical community watches with great interest. The results of this trial could pave the way for a new standard of care in cardiac surgery, transforming a routine procedure into an even more durable and effective treatment for heart disease.
Further Information and Resources
For those interested in the specifics of the trial and the science behind it, further details are available through the official university and NHS channels. This research not only highlights a potential breakthrough for patients but also showcases the leading role that UK institutions play in advancing global healthcare.
Discover more about the latest advancements in cardiovascular science and research at the University of Edinburgh’s dedicated Centre for Cardiovascular Science.
Learn more about the official announcement from NHS Glasgow and Greater Clyde regarding this groundbreaking trial.
Explore the work of the British Heart Foundation in funding critical research that translates scientific discovery into life-saving patient treatments.

